Let's All Remember When We Saved The World
To remind ourselves we can do it again.
Hello! This is Everything Is Amazing, a newsletter about science, curiosity and wonder.
Today, we’re coming full circle to finish the current season where we started it: in a runaway hot-air balloon about 35,000 feet (10.6 kilometres) above the English countryside, with one of its passengers unconscious from hypoxia and the other desperately tugging on the tangled release-line that has them soaring higher and higher…
Until all around them, the clouds have disappeared.
Over the last 4 decades of my life, I must have seen hundreds of cumulonimbus, billowing up into that classic mushrooming “thundercloud” shape with its anvil-like top (above).
And the whole time, I’ve looked up and thought wow, it must be super windy up there, to flatten it out like that.
This seemed logical to me. I mean, look at all those thin, whispy clouds further up, splayed across the sky like tattered streamers. Look how streaky they are! They must have been absolutely shredded. Yikes.
In fact, there are indeed some tremendously energetic winds around the height that Coxwell & Glaisher reached, called jet streams. During World War II, Japan tried to use one of them to waft around 9,300 incendiary balloon bombs across the Pacific and down onto the United States. While the assault was an almost complete failure, one bomb landed in a forest in Oregon and killed 6 people - the only fatalities from Axis action in the continental United States during the whole war, and the first deaths caused by an intercontinental weapon.
But generally speaking, jet streams are not why thunderclouds flatten out like that.
Were Coxwell and Glaisher to have failed to halt their ascent through the tropopause (the upper limit of this layer of thick, deliciously warm air we spend most of our lives at the bottom of) and into the vastly thinner stratosphere, in their last moments alive they would have entered an eerie, dry stillness - a serene graveyard for the two Englishmen, until the upper tropopause regained a hold on their balloon and gravity dragged their remains out the sky.
Weirdly, it wasn’t as cold up there as it should have been.
Throughout their ascent, the temperature had been falling away, past freezing-point, a long way past bearable, and to the point where Coxwell’s hands were uselessly dead to him.
But if the aeronauts had somehow both survived and been able to drive the balloon higher and higher, they’d have found that bitter temperature stabilizing, then reversing - until, around 50 kilometres above the ground, the air would have warmed to a relatively balmy −15 °C (5.0 °F).
This temperature gradient inversion - where the thin air is getting warmer with height, rather than colder - is the reason thunderclouds pancake at their tops. Down here near the ground, warm air is less dense than cold air and rises, which is how clouds drag their moisture into the sky. But in the stratosphere, air temperature increases with altitude, so any air trying to move upwards will be cooler than what’s above it - and will sink back down again.
Some thunderclouds still have enough energy to punch their way up into the stratosphere - but without convection doing its normal thing, none of that moisture can linger up there for long. Down it comes, or sideways it’s smeared, giving those rain-laden cumulonimbus clouds their classic hacked-off shape, and creating a place for airliners to avoid both turbulence and weather in general.
(Ever wondered why modern flights spend so much time at 35,000 feet? It’s to keep passengers happy in these silky-smooth conditions - and to use the thinness of the air to minimise drag and preserve fuel.)
In the words of Simon Clark (whose brilliant book Firmament I’m once again plundering here):
“Compared to the dense, humid, three-dimensional troposphere, the stratosphere is a ghostly realm. I think I find it, and Coxwell and Glaisher’s unintentional voyage into it, so fascinating because it is so close to us and yet so completely alien. If the stratosphere were at a horizontal distance rather than a vertical one, you could drive a car there in a matter of minutes, and on arriving you would be confronted by a realm of flat, arid motion. Yet for thousands and thousands of years, humans had no idea that their thin shell of an atmosphere was bounded above by this aeronautical desert.
We are surrounded by an alien world for our entire history and yet had not the slightest clue it existed.”
But now we know - because it’s the place where we saved the world.
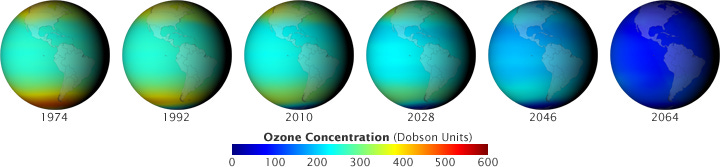
In the early 1970s, chemistry professor F. Sherwood Rowland and postdoc student Mario Molina were looking at the behaviour of chlorine atoms found in chlorofluorocarbons - non-flammable and low-toxicity chemical compounds that were considered safe enough to use in aerosol hairsprays and deodorants and, since the 1930s, millions of refrigerators worldwide, most famously under the brand name Freon.
To Rowland’s and Molina’s horror, their research suggested that once these gases dispersed into the upper atmosphere, every single chlorine atom had incredible destructive power in a very specific way: it could pull apart up to 100,000 atoms of ozone (O3).
If you’ve ever been reckless enough to go for a walk as a thunderstorm approaches, you might have noticed a coppery, vaguely bleach-like smell in the air. That’s ozone, dragged down from higher altitudes by the enormous downdraft of air that comes before a storm, and giving you a sense of what the stratosphere would smell like if your nose didn’t immediately freeze solid, which it absolutely would.
There’s not an awful lot of ozone up there, though. Most oxygen atoms in the stratosphere are of the O2 variety, and this is how ozone is created: incoming ultraviolet light from the Sun breaks apart O2 to create two O atoms, both of which can now combine with more O2 to make O3. It’s an elegant natural chemical factory working tirelessly above our heads.
Without it, we’d be in real trouble. And by “we”, I mean “all life on this planet,” because stratispheric ozone’s great gift to the Earth is that it absorbs huge amounts of UVB, the type of light that gives you sunburn, triggers skin cancer, and has the ability to damage materials, crops, marine organisms and even DNA itself.
(It’s also why the stratosphere gets warmer as you get higher.)
I know the title of this particular newsletter, ‘Let’s All Remember When We Saved The World’, is the kind that will attract clap-backs like “oh stop with the sensationalising, the ‘world’ will be fine, it’s just humans who will find it harder, I hate you climate-change clickbait people.”
To pre-empt a bit of that, let’s look at our world without its ozone layer, which was exactly what Rowland and Molina’s work seemed to predict.
Here’s science journalist and author of the newsletter The Science of Fiction Maddie Stone on just how bad it might get:
“By the middle of the 21st century, computer models suggest that in the world we avoided, global ozone levels would decline nearly 70 percent, doubling the intensity of UV radiation at Earth’s surface.
Rates of skin cancer and cataracts—responsible for about half of all blindness worldwide—would soar. The extra dose of UV would damage crops, potentially leading to global food shortages. And it would have a cascade of devastating effects on wild plants and animals which, like us, have evolved to survive in the low-UV conditions created by the ozone layer.
Widespread vegetation die-backs could ensue, triggering huge releases of carbon into the atmosphere and worsening global warming. (To add insult to injury, all the extra UV radiation would probably speed up organic matter decay.) Not even synthetic building materials would be safe from rapid deterioration under the harsh new Sun.”
I mean, that certainly meets my definition of “world-ending”?
Since sun tans have become a thing Westerners spend lots of time and money to acquire - or to fake - perhaps a statement like “by 2065 you could get sunburn in Washington DC in less than 5 minutes” doesn’t have quite the power to shock as it should. But sun tanning is damage - and no, a tan doesn’t protect you from sunburn, because a “base tan” is only like wearing Factor 4 sunblock.
(I wish I could tell all this to the Me of 20 years ago, who went walking on Crete without slapping on the right sun-cream, because these days both the sides of my face are blotched with unsightly hyperpigmentation. It’s no fun, believe me.)
Rowland and Molina published their findings in Nature in 1974 (and would share a Nobel Prize for it in 1995)- and immediately everyone firmly agreed that it was time to act! That’s how the real world works, right?
Uh…
“Industry representatives fought back: At one point, Aerosol Age, a trade journal, speculated that Rowland was a member of the Soviet Union’s KGB, out to destroy capitalism. Even some fellow scientists grumbled that he was going overboard with a hypothesis.” - UC Irvine News
“The Rowland-Molina hypothesis was strongly disputed by representatives of the aerosol and halocarbon industries. The chair of the board of DuPont was quoted as saying that ozone depletion theory is “a science fiction tale...a load of rubbish...utter nonsense”. Robert Abplanalp, the president of Precision Valve Corporation (and inventor of the first practical aerosol spray can valve), wrote to the Chancellor of UC Irvine to complain about Rowland’s public statements.” - Sharon Roan, Ozone Crisis: The 15-Year Evolution of a Sudden Global Emergency, via Wikipedia.
I guess that DuPont chair needed to read better science fiction!
I’m not a big fan of dystopian stories. For starters, they tend to brush over the extremely messy and uncertain parts of their histories when all hope is not yet lost and where people are working incredibly hard to move the needle in the right direction. You know, the exciting bits!
(I personally can’t think of a more thrilling time to base a story within, with more lessons to teach in that special way fiction can without you feeling you’re being schooled.)
Here’s science fiction author Kim Stanley Robinson being interviewed by Polygon:
“Anyway, science fiction is a modeling exercise where all the science fiction put together, especially all the near-future visions, they range from totally horrible to perhaps quite nice. It’s heavier-weighted at the disaster end than at the utopian end, maybe because it’s easier, or maybe because it’s more shockingly interesting to read. It’s not like going to town meetings and reading blueprints for plumbing facilities. The utopian end of science fiction has a reputation for being a dull, eat-your-greens type fiction, so there’s less of it compared to the disaster stuff. But there is both. And if you read a lot of it, one hopes you’re prepared for anything.”
Midway through his multi-award-winning Mars trilogy, leaders of various factions and settlements of the Martian underground come together in Dorsa Brevia, a 40-km-long ancient lava tube turned settlement, to try to thrash out via political consensus a working draft for a Martian Constitution.
The stakes are high: the clock is already ticking down towards a second planet-wide revolution against Terran (Earth) control, after the first accomplished little more than widespread chaos and bloodshed - and yet so many participating groups bitterly disagree on so many things. Can they come to a common understanding before it’s too late?
I really love this part of the story, and I’m not the only one:
Could such a thing happen in real life?
I reckon the Montreal Protocol on Substances That Deplete the Ozone Layer - signed 16th September 1987 and entering into force on January 1st 1989, to become the first universally ratified treaty in the entire history of the United Nations - answers this question with a firm Yes - and then adds that you don’t even need to get everyone together in one place to do it.
Much smarter people than I have spent the last 2 decades trying to understand exactly why it was such a resounding success, and let’s be clear here, I am just an idiot with a newsletter. But a couple of details stand out:
The agreement didn’t wait for all the science to be completely firmed up before implementing regulation - which is a good job, because early conclusions about ozone depletion levels were significantly underestimated. Instead, it adopted a “Precautionary Principle” that was enshrined in the Rio Declaration in 1992 - acting on likely evidence to avoid consequences that may be catastrophic or even irreversible if any delay is sought. (This is markedly different from how some politicians seem to think science should work - if their words can be believed, of course.)
Negotiations took place in small, informal groups, to give everyone the best chance of being heard and being understood. More than anything else, this reminds me of Dorsa Brevia, and how utterly exhausting that conference was for all the characters involved. Who knows how many such talks led to Montreal being accepted? But every one of them counted.
There was a clear economic benefit for the industries using CFCs to move away from them - not just on principle or to avoid public backlash, but because CFCs were old tech and therefore out of patent, and shifting to new alternatives would allow companies to develop ozone-friendly chemicals they could stick a profitable patent on.
And so the world was saved - just in time for its next challenge.
Now our climate is warming to dangerous levels and 2025 is on track to be the second or third warmest year on record (with 2024 being the warmest, thanks to El Niño). So what are we going to do about it?
This is a challenge on another scale. And instead of aerosol and halocarbon industries digging their heels in, it’s fossil fuel companies, some of the richest on the planet. And now we have opinionated billionaires in charge of social media platforms that over half of the world’s population uses.
Plus, the current President of the United States just stood in front of a United Nations assembly and called climate change a “con job”. Clearly this is not going to go smoothly.
At the same time, worldwide adoption of so-called ‘clean energy’ (sources that don’t emit greenhouse gases like carbon dioxide or pollute the environment, although they do currently require non-renewable materials to be built) is rocketing right now. The pace of adoption, particularly of solar power and particular in China (which is responsible for around 25-30% of CO2 emissions worldwide), is truly awe-inspiring, as Sam Matey-Coste explains here.
Equally remarkably, this just happened:
But earlier this year, environmental journalist Bill McKibben added a note of caution against complacency in an interview with Emily Atkin’s newsletter HEATED:
“Forty years from now, we’re going to run the whole world on sun and wind... But if it takes us anything like 40 years to get there, forget it. The world that we run on sun and wind is going to be broken.”
Like the Montreal Protocol and the Paris Agreement of 2016 (and the fictional Dorsa Brevia Declaration), it’s a race with everything at stake. And it’s going to be difficult as hell to throw together something that everyone can agree on. Whether it will happen or not in time to avoid the worst disruptions to our fragile atmosphere and everything underneath it, that’s still unclear at this point.
But can it happen? Can we work together and act in time?
I think based on our recent history, those questions already have an answer.
Images: CALIPSO>CALIOP/NASA Visible Earth; Steve & Barbe Sande; NASA/Reid Wideman; UC Irvine.








I was a teenager during the ozone scare and Montreal resolution... it was HUGELY impactful on me, and forever on my own thinking as a climate advocate. It was profoundly inspiring to see the world come together to solve that problem. I keep hoping we'll do it again in time (and listening to Bill McKibbon again recently made me believe we can, since most of the world is actually on board now... just need to get my big dumb country back on track!)
Your writing and reporting are powerful and important. I want you to have a very large platform for it!